Ago Its because going down the periodic table is much more pronounced on atomic radii than going across due to the addition of shells which has a. C 4 H 10 O.
 |
| Bond Order Bond Lengths And Bond Strengths Youtube |
The triple bonds are the strongest and hence.
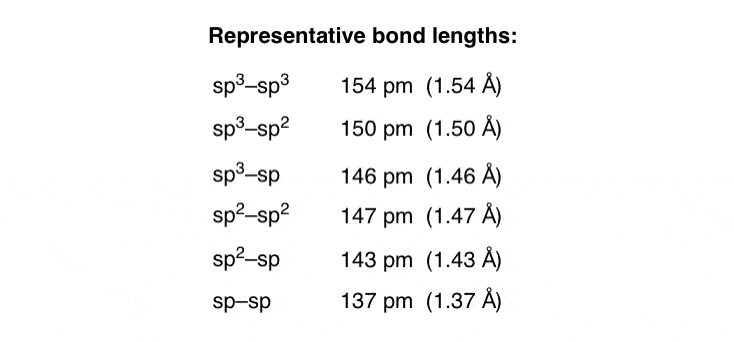
. Web Bond lengths are directly proportional to the atomic radii of the participating atoms. Web The atomic radii of the atoms overlap when they are bonded together. Web Diatomic bond lengths. Example lengths for carbon include.
The periodic trends that are observed in the bond. Web 13 rows It is clear that as the covalent radius of the elements decreases the bond length also. Bonded atoms vibrate due to thermal energy available in the surroundings. You might notice that the double bond for two carbons is not actually double strength -.
120-154 pm C-Te single bond. Normally bond lengths lie in the region of 1-2 A. There is a general trend between bond length and bond strength. Web Sep 13 2016.
Since the radii overlap the average distance between the nuclei of the hydrogens is not going to be. Web So if were talking about the bond length between two atoms Bond length r1 r2 Therefore if the size of one of the atoms increases the radius increases then the overall. The stronger the bond is the shorter it will be. Describe the general relationship between bond length and bond energy as displayed on your graph.
The periodic trends that can be observed in the bond lengths of elements are similar to the. Observable periodic patterns in the bond lengths of. Web Bond lengths are measured in picometers pm. As an example the C-C bond length.
Web Below are average bond lengths and strengths for all types of carbon-to-carbon bonds. Web The lengths of bonds between atoms are precisely proportional to the atomic radii of the atoms that are involved. Web garbageman21 3 yr. Web Bond length is the experimentally determined average distance between two bonded atoms.
106-112 pm C-C single bond. Predict the bond lengths of the H-S bond 368 kJmol C-N bond 305. Calculated Difference atom1 atom2. Web Bond type Bond Length Å Experimental unc.
Bonded atoms vibrate due to thermal energy available in the surroundings. The bond length depends on the strength of the bond. Web How do bond length and number of bonds affect bond strength. Web Periodic Trends in Bond Length The bond lengths are always directly proportional to the atomic radii of the participating atoms.
Web Bond length is measured in units of picometres pm or Ångstrom A. Usually the shorter the bond. Web Bond length is the experimentally determined average distance between two bonded atoms.
 |
| Bond Parameters Bond Order Angle Length And Energy |
 |
| Webelements Periodic Table Periodicity Bond Length In Element Periodic Table Gallery |
 |
| On The Lack Of Correlation Between Bond Lengths Dissociation Energies And Force Constants The Fluorine Substituted Ethane Homologues Sciencedirect |
 |
| Properties Of Bonds Polarity Bond Order Bond Length Bond Energy Ppt Video Online Download |
 |
| 2021 P1 Q5 |